[PHAICELL] Coherent microscopy with a quantitative phase contrast: a return to the basics and a proposal of a new technique of numerical reconstruction, applied in advanced biomedical imaging without markers
head of the project: Maciej Trusiak, PhD, BEng
task duration: 19.01.2021- 18.01.2025
funds for the project: 1 999 200 PLN
When compared to other modern techniques of imaging without markers, the Quantitative Phase Microscopy (QPM) is an extremely efficient method, featuring high contrast. “Without markers” implies that the sample is not subjected to colouring or fluorescent marking and that it may be imaged based on the endogenous contrast measures, i.e. the light refraction coefficient. (which introduces the measurable varied delay of the optical phase). This non-phototoxic, non-invasive imaging method brings biology and metrology together since it generates a quantitative map of a living biological sample (cellular mass, volume, surface and their evolution in time), upgrading the sample visualisation for its marker-less optical measurement, enabling precise diagnostics.
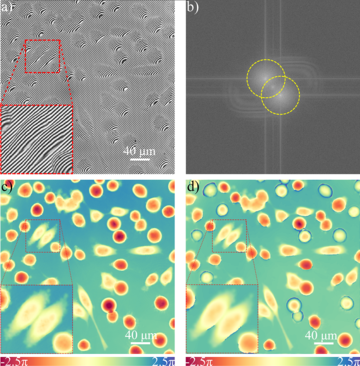
Fig. 1: QPM studies of HeLa cells:(a) an interferogram recorded in Linnik microscope system, (b) an interferogram spectrum with troublesome overlapping (c) a reconstruction of quantitative phase distribution using numerical super-resolution method developed at the Warsaw University of Technology, (d) a reconstruction, much poorer in details of cell structure, performed with the traditional Fourier transformation method (loss of phase resolution is caused by partial overlap of information in the spectrum). The research was performed in collaboration with The Arctic University of Norway (group of Balpreet Ahluwalia, Prof.Tit).
The issue we would like to tackle as a part of the PHAICELL project is connected with the fact that QPM rapidly entered the development phase aimed at application while there are still fundamental limitations of the quantitative phase imaging that need to be examined. Moreover, based on the research on these limitations, new techniques of numerical reconstruction of a phase map, enabling us to overcome selected obstacles in the now-available QPM systems. These include a limited information content, among others. Aside from these fundamental numerical research, new experimental applications of the method will be tested during the PHAICELL project.
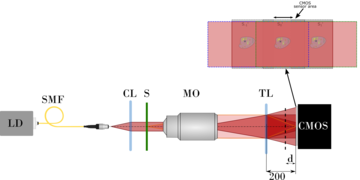
Fig. 2. A schemata of a PWskope – a modern microscope designed based on the common path interferometer that utilises a diffractive network for an achromatic generation of quantitative phase contrast.
We estimate that this research work will enable the devising of imaging tools, allowing for significant progress in biomedicine, and in particular in the following areas:
- in neurobiology and stem cells analysis, which we plan to research with our partners from the Institute of Experimental and Clinical Medicine (IMDiK);
- an analysis of sperm, conducted in cooperation with our partners at the University of Valencia (UVLC);
- in research on fish cells polluted with microplastics, conducted with our partners from the Arctic University of Norway in Tromso (UiT).
Fig. 3. Author's arrangement of a Fourier ptychographic microscope performing QPM measurements with numerically increased resolution while maintaining a wide field of view. The results of amplitude imaging for basic resolution resulting from lens parameters and phase imaging results for ptychographically increased resolution (the object of research were buccal epithelial cells) are presented.
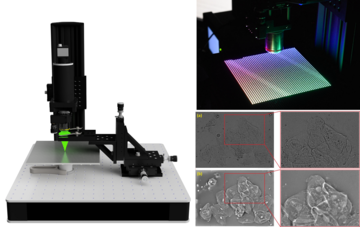
The common works with IMDiK will be conducted with the use of a modified matrix phase microscope of a common way, the so-called PWskop 2.o. The original version of this groundbreaking microscope was built at the Institute of Micromechanics and Photonics of PW during the very successful OPUS 13 project (head of the project: Maciej Trusiak). It is a unique piece of equipment on a worldwide scale and is extremely compact. As a part of the PHAICELL project, our plan is to modernise the PWskop in respect to its numerical and research capabilities, build its prototype with a 3D printer and install it at UiT and UVLC to enable local research on interesting biological samples.
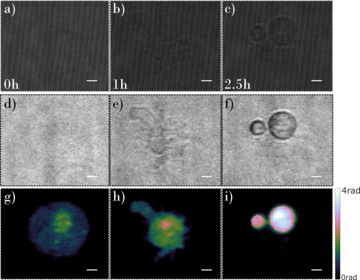
Fig. 4: A time-lapse study of a dying mesenchymal stem cell using the PWscope: (a)-(c) recorded interferograms, (d)-(f) calculated amplitude distributions (corresponding to cellular absorption), (g)-(i) quantitative phase contrast distributions (corresponding to cellular structure refractive index distribution); images (a) (d) (g) were recorded at the beginning of the study, images (b) (e) (h) were recorded after one hour of study, and images (c) (f) (i) were recorded after 2.5h. The study was performed in cooperation with the Institute of Experimental and Clinical Medicine of the Polish Academy of Sciences (group of Barbara Łukomska, Prof.Tit).
In the PHAICELL project, our goal is to research, define and exceed the boundaries of Quantitative Phase Microscopy in the scope of rapid imagining under the regime of limited budget of photons, extensive blurr of registered interferograms and a strong absorption of biological objects. We have noted a need for filling in the designated gaps in the QPM technology across the world. This is further underscored by a growing number of scientific publications in prestigious periodicals with an extensive audiences and the popularity of commercial products (Nanolive, for instance). PHAICELL will utilise advanced techniques both in the experimental works, i.e. the application of pseudo-thermal light sources, specifically engineered waveguides and the PWskop 2.0 unique phase microscope; and in the numerical actions, i.e. the application of a new ne adaptive local iterative filtering and variable decomposing. Owing to this innovative methodology, PHAICELL will strive to significantly advance the current state of knowledge in QPM and enable rapid, non-invasive biological imaging under the conditions of low light intensity, limited signal and an increased precision of the phase contrast. Our partners from the area of biomedicine will be there to make sure that the ambitious applications are up to date.
Fig. 5: Studies on gold nanoparticles (100nm diameter): (a) interferogram spectrum with traced numerical aperture and clearly marked aliasing (overlap) of spectral information, (b) phase distribution obtained using traditional Fourier transformation method, (c) phase distribution obtained using author's Hilbert-Huang method, (d) phase distribution obtained using author's variational Hilbert transform method. The improvement in the resolution of quantitative phase imaging is significant and strongly dependent on the phase map reconstruction algorithm (the same interferogram was studied). The research was carried out in collaboration with The Arctic University of Norway (group of Balpreet Ahluwalia, Prof.Tit).